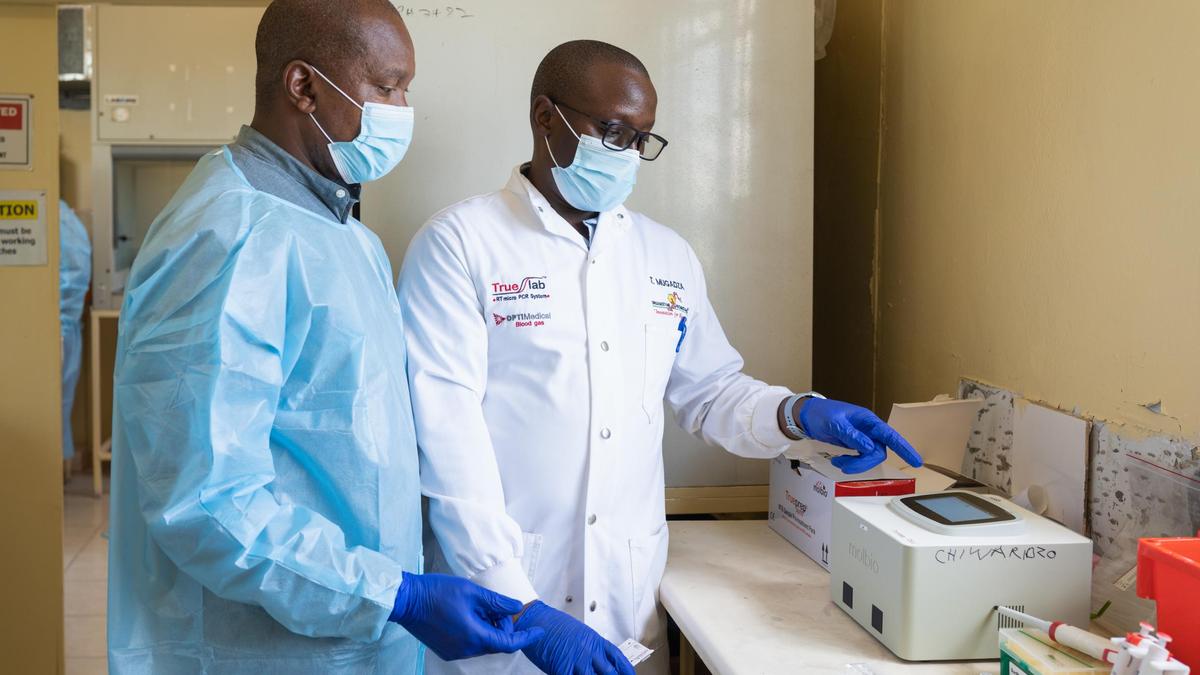
Looking back at the history of tuberculosis (TB) control, one small yet revolutionary change stands out — the development of point-of-care molecular diagnostics. Traditionally, TB diagnosis involved either a poorly sensitive sputum smear test or waiting for weeks for culture results from centralised labs. However, with small, battery-powered Polymerase Chain Reaction (PCR) machines, both TB and drug-resistance can now be diagnosed in under an hour.
A diagnostics game changer
World Health Organization (WHO)-approved rapid molecular diagnostic platforms such as Truenat are now a cornerstone of the worldwide fight against TB, particularly in low-resource settings. For instance, after integrating it with the Nigerian national TB programme, the identification of rifampicin-resistant TB cases nearly doubled, which was an indicator of the platform’s ability to detect drug-resistant strains. In addition, Nigeria has experimented with stool-based Truenat testing for the diagnosis of TB among children in primary health-care centres. This is intended to overcome the challenge of sputum collection among children, thus improving case detection and bacteriological confirmation.
A study conducted recently and published in The Lancet assessed the use of this platform in primary health-care centres in Mozambique and Tanzania, in Africa. The trial revealed that combining on-site molecular testing with rapid communication of results greatly improved the rate at which patients began treatment within seven days of their initial visit. The intervention not only improved diagnostic quality but also optimised treatment, proving the instrumental role of point-of-care diagnostics in improved TB care in resource-constrained environments. Such innovations are critical to the achievement of global TB elimination targets.
Editorial | Swing, but do not miss: On India and the WHO’s Global Tuberculosis Report 2025
This shift in diagnostic capacity did not merely alter the rhythm of TB control. It became the basis for a world where early treatment was no longer a function of geography or infrastructure.
It was this very shift that was recognised globally this year, when Goa-based Molbio Diagnostics was awarded the coveted Kochon Prize for its contributions to TB diagnostics. Established by the Kochon Foundation of the Republic of Korea and in collaboration with the Stop TB Partnership, the Kochon Prize is one of the world’s most distinguished awards for TB control contributions. It has honoured scientists, organisations and individuals whose innovations have radically enhanced TB outcomes. The real value of this award transcends any single company. This year’s award says a lot about the extent to which India’s indigenous technological innovations are not just fighting TB within its own geography but also significantly influencing the world of TB eradication.
This writer recalls WHO’s endorsement, in 2020, of the portable molecular platform developed in India. The evidence came from diverse studies in Asia and Africa, showing an impact equivalent to central-lab systems but with significantly greater deploy-ability. The excitement was palpable — at last, a diagnostic test that could go to the patient, not the other way around. It was a moment when innovation met inclusion. Since then, many more Indian companies have ventured into TB diagnostics innovation, which is visible today in the range of improved point of care tests available.
India’s role in decentralised TB control
India’s National TB Elimination Programme (NTEP) was swift in adopting these technologies, installing thousands of point-of-care molecular testing units across the country. Today, we have enough and more evidence of how this has significantly reduced the time between suspecting TB and starting treatment, thereby greatly enhancing TB management protocols.
This award also highlights India’s collaborative model for TB elimination. Today, the TB fight is no longer solely the responsibility of the public sector. It is a shared mission, where private innovators, community health workers and government systems work hand-in-hand. For a country that bears close to a quarter of the world’s TB burden, this partnership is not only desirable, it is imperative. The private sector offers agility, scale and technology while the public sector offers reach, data and accountability. Together, they create the foundation of a strong public health response.
The Kochon prize has been awarded to India twice — in 2006 to Dr. L.S. Chauhan, Deputy Director General–Tuberculosis, Ministry of Health and Family Welfare (joint winner with TB/HIV activist Winstone Zulu from Zambia), and in 2017 to the Indian Council of Medical Research (ICMR). This year’s recognition is not just another win for India. It is a global acknowledgement of the power of affordable indigenous innovation.
The adoption of field-ready molecular diagnostics from India has led to deployments worldwide — from mobile clinics in sub-Saharan Africa to refugee camps in Eastern Europe. It proves that India can be at the forefront of innovation, creating scalable solutions that can transform global health equity.
But the fight against TB is not done yet. The next challenge lies in ensuring that such diagnostics are accompanied by balanced access to treatment, nutrition, social protection and reduction of stigma. Studies have established that malnutrition accounts for about 40% of TB in India. The battle against TB will have to address all of these determinants in order to succeed.
A call for continued investment, innovation
We stand at a critical juncture. While the recognition of innovations such as point-of-care diagnostics is heartening, we must not lose sight of the bigger picture: TB is a disease of inequality.
We must continue investing in innovations that integrate diagnostics with nutrition, contact tracing, digital adherence and vaccines. Only then can we ensure that no one is left behind in this global effort to eliminate TB.
Dr. Soumya Swaminathan is former Chief Scientist, World Health Organization
Published – November 20, 2025 12:08 am IST